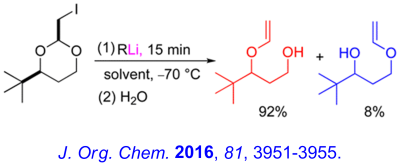
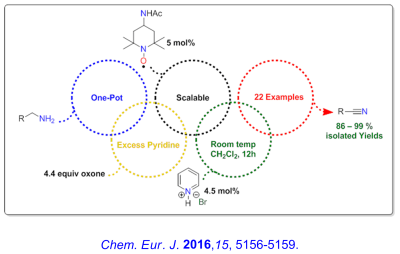
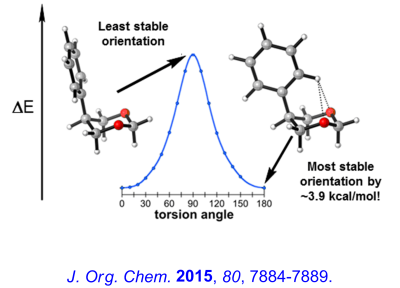
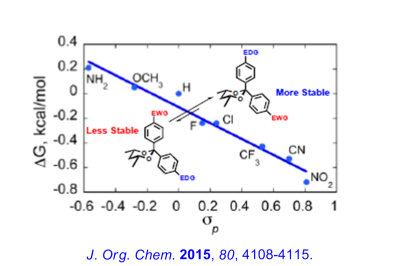
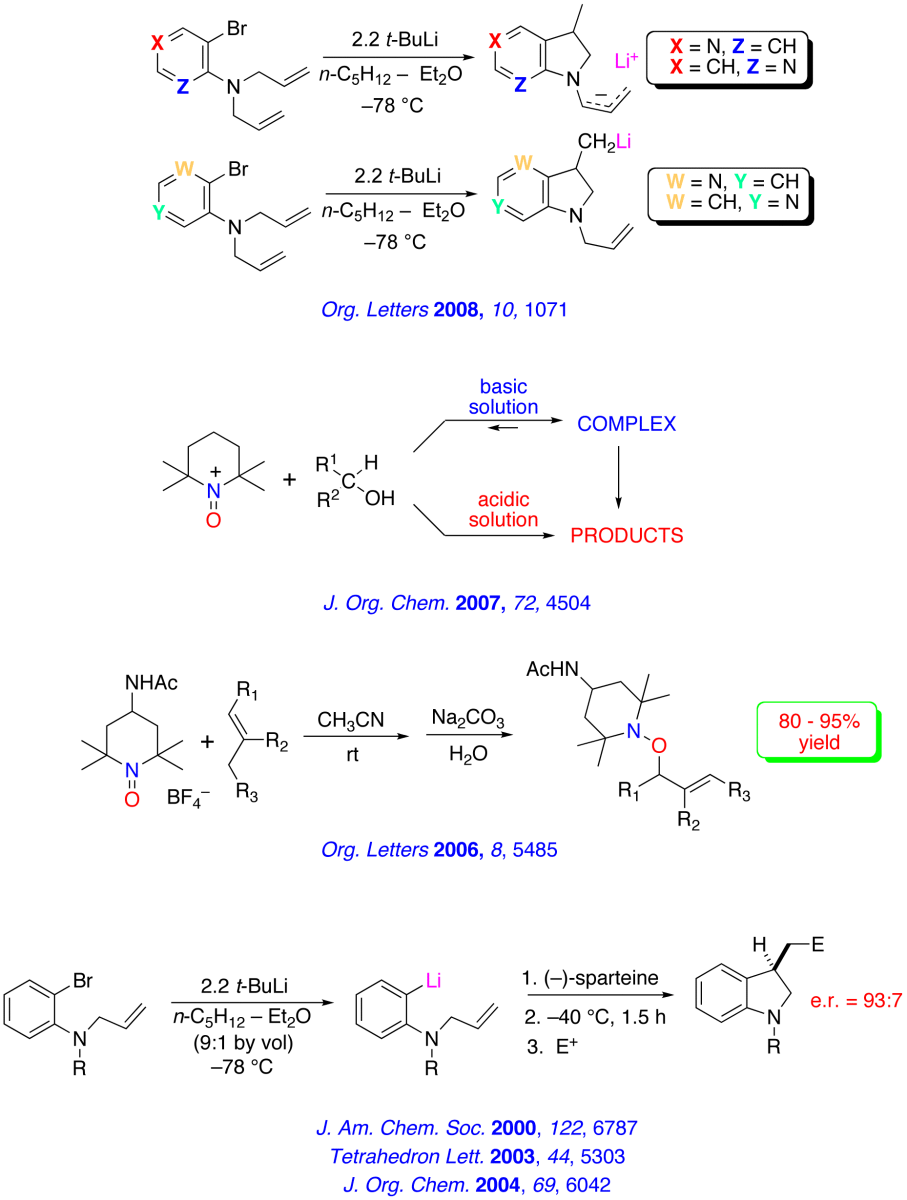
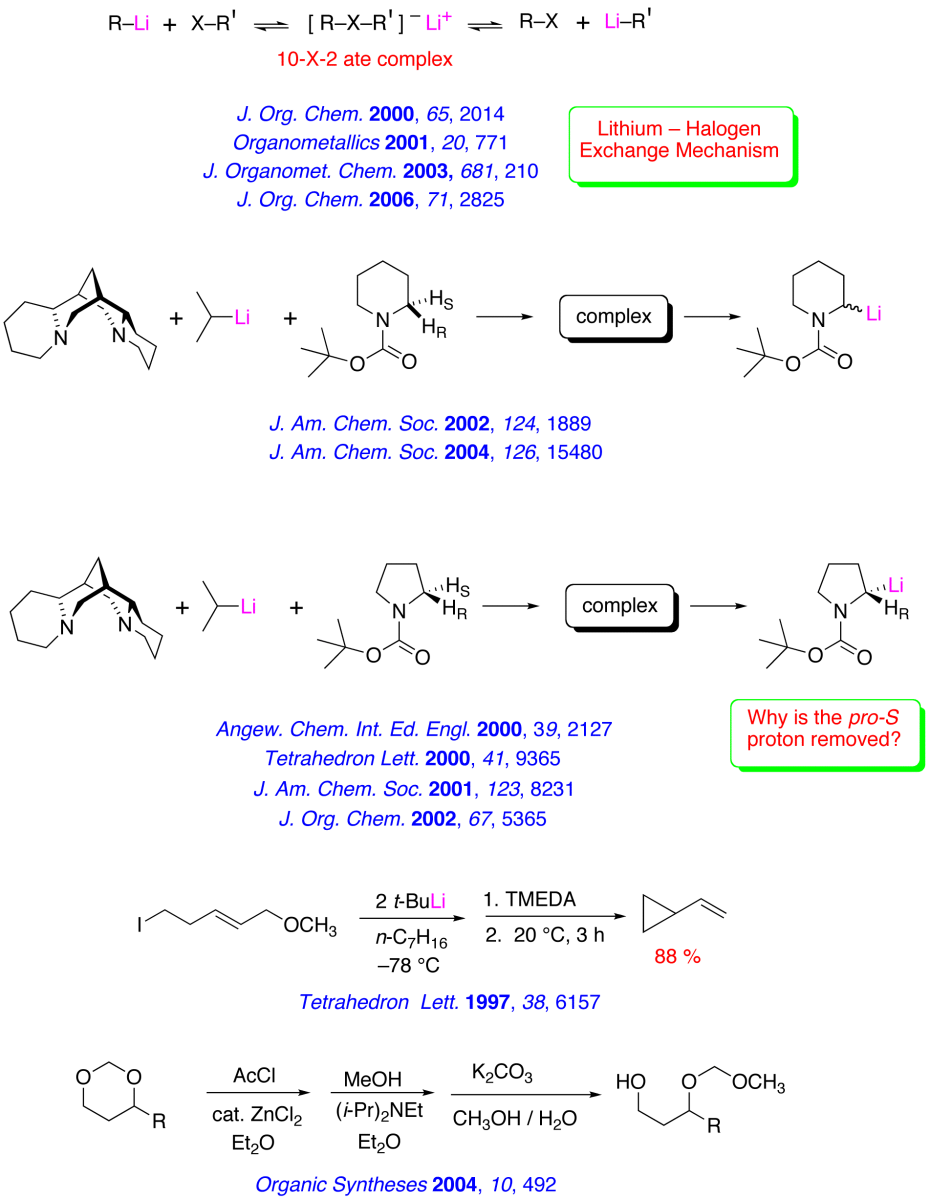
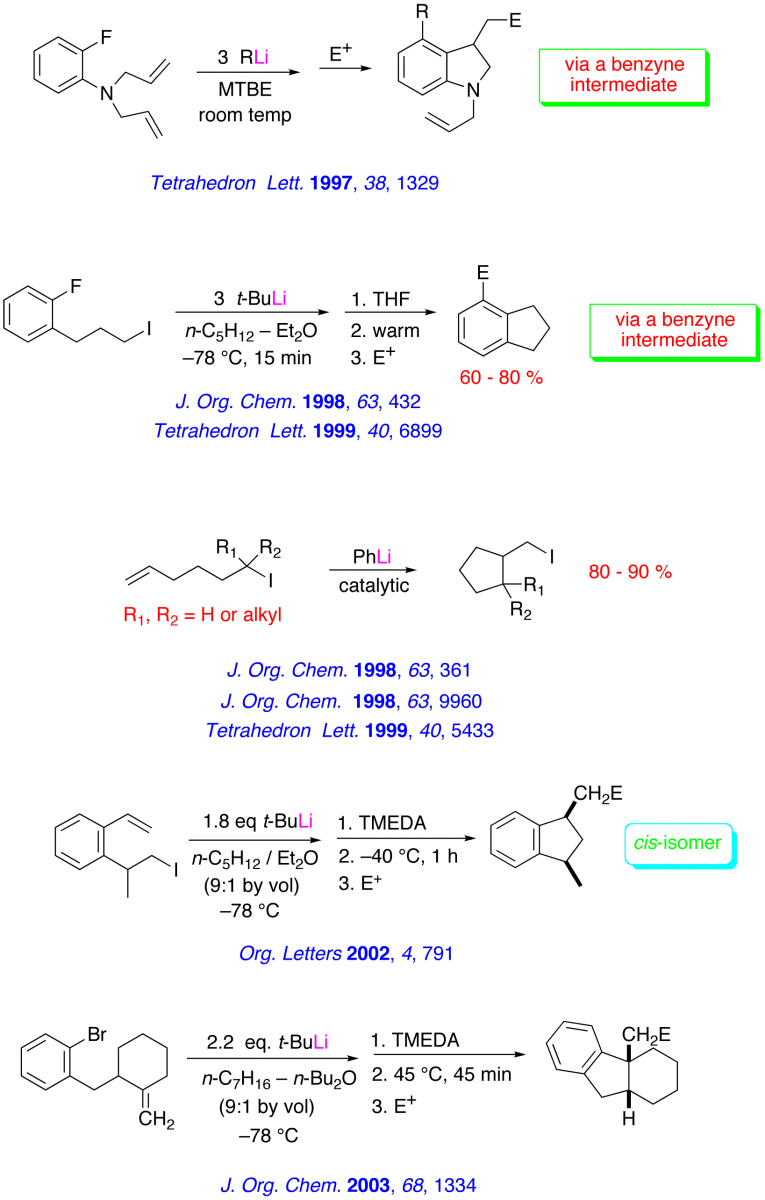
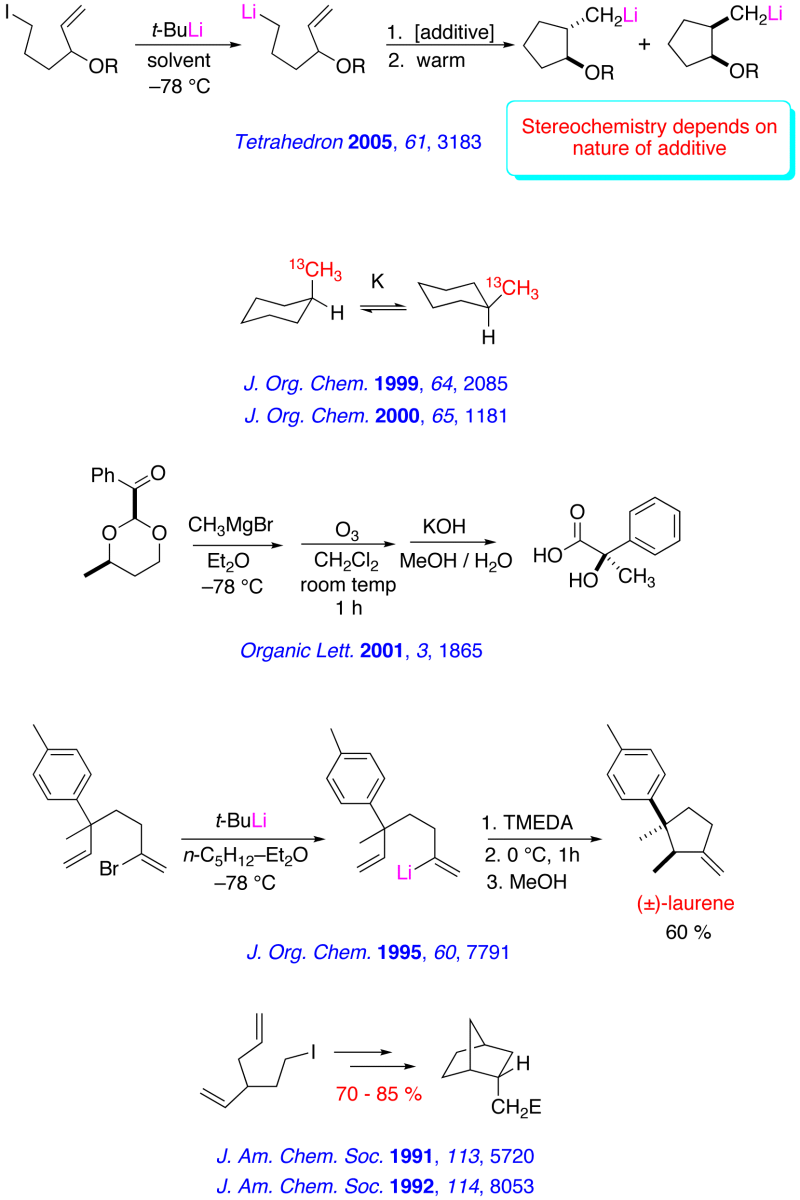
Recent Research in the Bailey Group
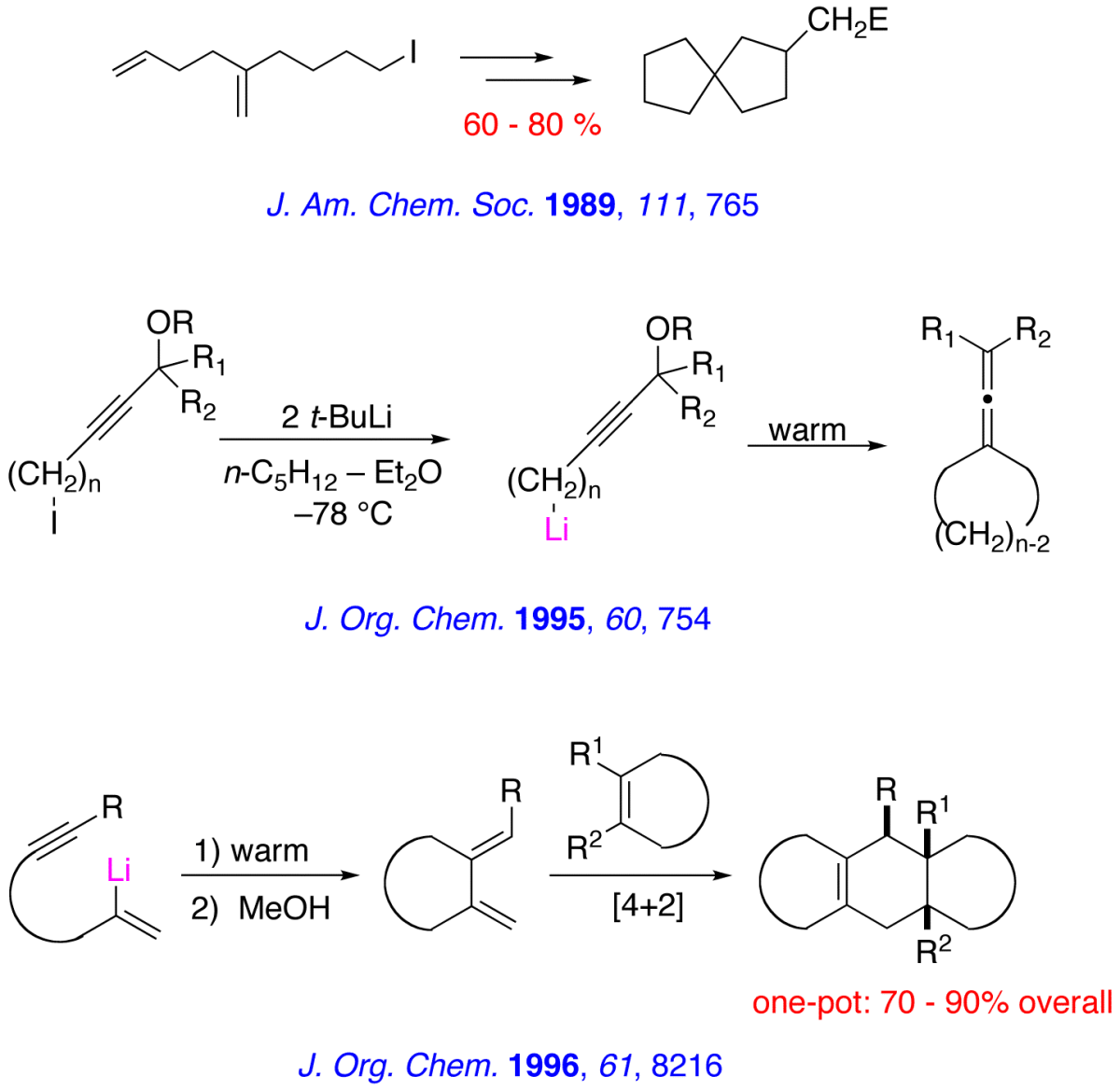
The major research interests of our group lie in the development of new synthetic methodology using novel main-group organometallic chemistry. A key focus of our effort involves investigation of the synthetic utility of unsaturated organolithiums and related Group 1 organometallics. These reactive intermediates, which we have found may be prepared in virtually quantitative yield by low – temperature lithium – iodine exchange between t-butyllithium (t-BuLi) and an appropriate organoiodide, undergo regiospecific and highly stereoselective cyclization upon warming. The high degree of stereocontrol inherent in these anionic cyclizations has led to development of one step, stereoselective syntheses of simple natural products and tandem – cyclization strategies for the preparation of bicyclic and polycyclic structures. A brief, schematic survey of some of our work in the area of intramolecular carbolithiation is presented below.
Enantioselective routes to a variety of carbocyclic and heterocyclic molecules, and natural products based asymmetric cyclization of achiral olefinic organolithiums that are controlled by a stereogenic lithium have been investigated. In parallel with our synthetic efforts, we are engaged in mechanistic, spectroscopic and computational investigations of the fundamental structure and reactivity of Group 1 organometallics.
Our group is actively exploring the oxidation chemistry of oxoammonium cations and we have a long standing interest in the investigation of reaction mechanisms and molecular structure and energetics. Currently, we are interested in developing efficient methodologies for amine oxidations and closely related functional group transformations.